Key interests
Current challenges that we are tackling include:
- Characterisation of how complement
opsonisation is regulated and what communicative signals complement
opsonins send out to bystander cells
- Development and testing of novel biopharmaceutical complement inhibitors
- Characterisation of inflammatory complement activation on bio- and nanomaterials
Investigation of complement's influence on shaping an inflammatory or a tolerogenic immune response Characterisation of the interactions between complement proteins with the malaria parasite Plasmodium falciparum
Main methods
We
specialise in characterising protein-protein, protein-ligand and
protein-cell interactions and furthermore engineer, produce and test
novel immunmodulatory biopharmaceutical candidates. To achieve
this we use a wide range of protein biochemical and immunological
techniques ranging from recombinant protein technology (protein
expression in bacterial, yeast and mammalian hosts followed by protein
purification), knowledge-based protein engineering, protein
chemistry, biophysical interaction analysis (e.g. SPR), fluorescent cell microscopy, fluorescence-activated cell sorting (FACS), protein and cell-based immunological assays (e.g. ELISPOT analysis).
Overview of the complement systemThe complement system is the main
soluble effector arm of innate immunity and is found ubiquitously in the human
body. Its omnipresence warrants fast and efficient immune surveillance and
additionally maintains vital host homeostasis. More than 30 soluble and cell-surface
anchored complement proteins cooperate to manage the central element of the complement
system, the complement cascade. The liver supplies the blood stream with large
amounts of complement components, but a huge number of cells and tissues add to
the systemically provided components through local production.
Owing to its phylogenetically long
and ubiquitous presence at high concentrations, complement’s role expanded from
an originally old, self-sufficient protection mechanism to an interconnected
player managing global immune surveillance and tissue homeostasis with such
diverse effects and influence on as:
defense against microbial invaders,
removal of cellular waste and debris (e.g. apoptotic cells), crosstalk with
Toll-like receptors, interplay with coagulation, enhancing humoral immunity,
regulating T-cell responses, inflammatory diseases and acute phase disorders
(for a detailed compendium see (Ricklin et. al., Nature Immunology, 2010)).
Three distinct pathways - the
classical, the lectin and the alternative pathway – trigger the complement
cascade intrinsically. Extracellular, soluble complement pattern recognition
molecules (of the classical and lectin pathway) serve as sentinels for danger
signals, and upon recognition of pathogen associated molecular patterns (PAMPs)
or endogenous danger associated molecular patterns (DAMPs) trigger distinct
complement activation profiles. In contrast, the alternative pathway (AP) is
not specifically activated by PAMPs or DAMPs, but is active at all time at a
very low level. However, host-specific regulator molecules control AP-activation
on self-cells, thus protecting from the AP via “missing-self recognition”. Complement
activation cumulates in the central step of the complement cascade, the
enzymatic activation of the pivotal complement protein C3 via convertase
enzymes into the small anaphylatoxin C3a and the 175 kDa opsonin C3b, which
indiscriminatingly attaches to nucleophils in its vicinity through its reactive
thioester forming a covalent bond. C3b-opsonised molecules, particles and
surfaces act, if not held in check by surface associated regulators, as germination centres for the
auto-amplification of further C3b opsonins through newly formed C3-convertases,
thus auto-amplifying the initial trigger, irrespective of its origin, by a
multifold.
Scheme of the major complement pathways:
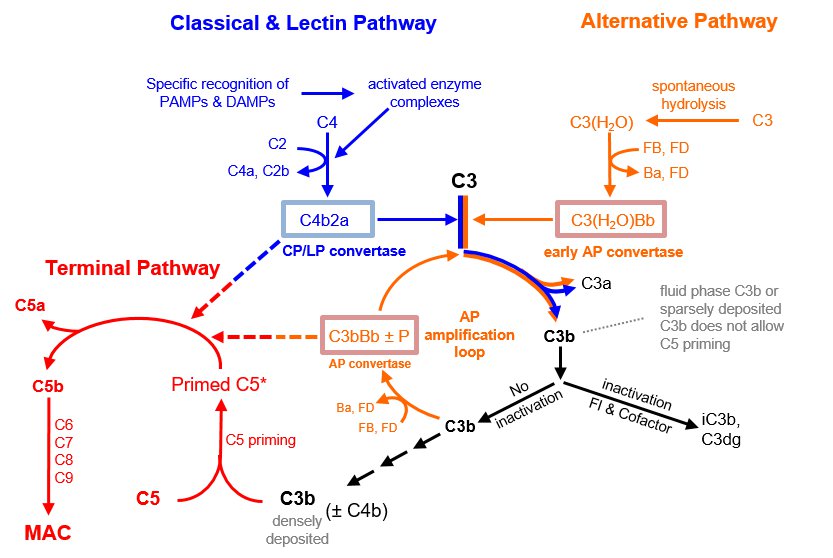
Modifed from Mannes et al. Blood. 2021;137:443-455 Tight regulation of the pivotal
C3-convertases – and in particular of the AP-amplification loop C3-convertase –
is of crucial importance and under-regulation of this central step leads to
severe tissue damage and is the underlying and/or aggravating factor of many
rare and common disease conditions such as e.g. paroxysmal nocturnal
hemoglubinuria (PNH), atypical haemolytic uremic sundrom (aHUS), age related
macuar degeneration (AMD), asthma, rheumatoid arthritis, systemic lupus
erythematosus, multiple sclerosis, Alzheimer’s disease, ischemia-reperfusion
injury, transplant rejection.
Complement activation must be spatially and temporarily limited in order
to avoid damage to normal self as a consequence of excessive widespread, and
prolonged activation (which also leads to exhaustion of complement proteins in
one event and consequently to unresponsiveness to later events).
Under normal, physiological
conditions the level of complement activation and associated immunological
outcome ideally is dependent on the nature of the initiating stimulus.
A) On pathogens complement acts as
the first responder of the immune system in a robust and unrestricted
activation profile promoting clearance, inflammation and consequently immunity:
Insufficient regulation of the
C3-convertases on pathogens leads to massive C3-opsonisation, followed by the
assembly of C5-convertases, which process the complement C5 protein into the
very potent anaphylatoxin C5a and C5b. While C5a promotes inflammation, mast
cell degranulation and leucocyte migration to alert all branches of the immune
system, C5b associates with the complement components C6, C7, C8 and multiple copies
of C9 to form the membrane attach complex (MAC) which punches holes into cells
eventually resulting in lytic cell death. (i.e. raising alarm within the
entire immune system).
B) On altered self (e.g. apoptotic
and injured cells, debris) complement promotes a limited, more regulated
activation profile resulting in mild inflammation, but preventing immunity and
thus supports house-keeping functions:
Intense regulation of the
C3-convertases stops the complement cascade from progression into the
detrimental lytic pathway, but allows – to varying extent - particles and
molecules to be coated with opsonin C3b and Factor I-processed inactivation
products of C3b: iC3b, C3dg, C3d. Coating with opsonins facilitates clearance
and uptake of antigens by professional phagocytotic cells, enhanced antigen
presentation, lowering of thresholds for B-cell activation (T-cell dependent
and independent B-cell activation).
C)
On normal self (e.g. healthy cells) complement activation is limited to
the baseline activation of the alternative pathway. In the presence of
regulators this low level surveillance-activation is insufficient to raise
inflammation and immunity.
|

